A thermodynamical and structural study on the complexation of trivalent lanthanides with a polycarboxylate based concrete superplasticizer†
Abstract
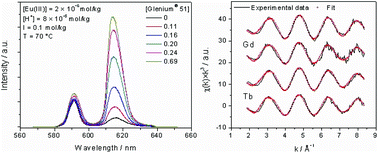
The complexation of trivalent lanthanides with a commercial polycarboxylate based concrete superplasticizer (Glenium® 51) is investigated using different spectroscopic techniques. Time-resolved laser fluorescence spectroscopy (TRLFS) in combination with a charge neutralization model is used to determine temperature dependent conditional stability constants (log β′(T)) for the complexation of Eu(III) with Glenium® 51 in 0.1 mol kg−1 NaCl solution in the temperature range of 20–90 °C. Only one complex species is observed, and log β′(T) (given in kg per mol eq) shows a very slight increase with temperature from 7.5 to 7.9. The related conditional molar reaction enthalpy (ΔrH′m) and entropy (ΔrS′m) obtained using the Van't Hoff equation show that the complexation reaction is slightly endothermic and entropy driven. The thermodynamic investigations are complemented by structural data for complexes formed with Gd(III) or Tb(III) and Glenium® 51 using extended X-ray absorption fine structure (EXAFS) spectroscopy. The results imply a non-chelate coordination of the trivalent metals through approximately three carboxylic functions of the polycarboxylate comb polymer which are attached predominantly in a bidentate fashion to the lanthanide under the given experimental conditions.


 Please wait while we load your content...
Please wait while we load your content...