Magnetostructural relationship for μ2-phenoxido bridged ferric dimers†
Abstract
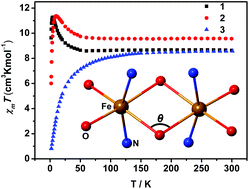
Three Fe(III) dimers, [Fe2(L-H)2]·2CH3CN (1), [Fe2(L-OCH3)2] (2) and [Fe2(L-OC2H5)2]·2CH3CN (3), containing the pentadentate O,N,N,O,O-donor Schiff-base ligands with variable size pendants, were synthesized and structurally characterized. The three ligands were generated in situ from 2-(iminomethyl)phenol, 2-methoxy-6-(iminomethyl)phenol and 2-ethoxy-6-(iminomethyl)phenol, respectively. All three crystal structures contain centrosymmetric dimers of edge-sharing octahedra of Fe(III) ions through a pair of μ2-phenoxido bridges. The exchange coupling is ferromagnetic for 1 (J = +0.47(1) cm−1, ∠Fe–O–Fe = 98.02°) and 2 (J = +0.86(1) cm−1, ∠Fe–O–Fe = 97.17°), but antiferromagnetic for 3 (J = −0.72(1) cm−1, ∠Fe–O–Fe = 98.53°), which are correlated by high-field electron paramagnetic resonance revealing moderate magneto-anisotropy of D = −0.24(3) cm−1, E = 0.08(1) cm−1 for 1, D = −0.38(1) cm−1, E = 0.11(1) cm−1 for 2, and D = 0.30(3) cm−1, E = 0.02(1) cm−1 for 3. The exchange couplings were further estimated by DFT calculations, which gave the finest Fe–O–Fe angle of 97.83° for the ferromagnetic–antiferromagnetic crossover.
- This article is part of the themed collection: Dalton Transactions Inorganic Symposia


 Please wait while we load your content...
Please wait while we load your content...