Raman spectroscopic characterization of light rare earth ions: La3+, Ce3+, Pr3+, Nd3+ and Sm3+ – hydration and ion pair formation†
Abstract
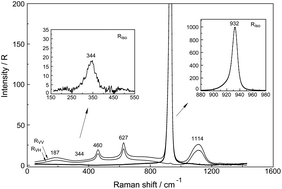
Raman spectra of aqueous La3+, Ce3+, Pr3+, Nd3+ and Sm3+ – perchlorate solutions were measured and weak strongly polarized Raman bands were detected at 343 cm−1, 344 cm−1, 347 cm−1, 352 cm−1 and 363 cm−1, respectively. The full width at half height for these bands is quite broad (∼50 cm−1) in the isotropic spectrum and the band width increases with increasing solute concentration. The polarized Raman bands were assigned to the breathing modes of the nona-aqua ions of the mentioned rare earth ions. Published structural results confirmed that these ions exist as nona-hydrates in aqueous solutions [Ln(H2O)9]3+. The Ln–O bond distances of these rare earth ions correlate well with the band positions of the nona-aqua ions [Ln(OH2)9]+3 (Ln = La3+, Ce3+, Pr3+, Nd3+ and Sm3+) and the force constants were calculated for these breathing modes. The strength of the force constants increase with decreasing the Ln–O bond distances (La–O > Ce–O > Pr–O > Nd–O > Sm–O). While the fully hydrated ions are stable in dilute perchlorate solutions (∼0.2 mol L−1), in concentrated perchlorate solutions outer-sphere ion pairs and contact ion pairs are formed (C > 1.5 mol L−1). In a hydrate melt at 161 °C of Ce(ClO4)3 plus 6H2O, the contact ion pairs are the dominate species. The Raman bands of the ligated perchlorate and the Ce–O breathing mode of the partially hydrated ion pair at 326 cm−1 were measured and characterized. In cerium chloride solutions chloro-complex formation was detected over the measured concentration range from 0.270–2.167 mol L−1. The chloro-complexes in CeCl3(aq) are weak and diminish rapidly with dilution and disappear at a concentration <0.1 mol L−1. In a CeCl3 solution, with additional HCl, a series of chloro-complex species of the type [Ce(OH2)9−nCln]+3−n (n = 1, 2) were detected.


 Please wait while we load your content...
Please wait while we load your content...