Hydroxide-bridged dicopper complexes: the influence of secondary coordination sphere on structure and catecholase activity†
Abstract
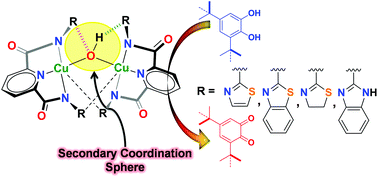
Amide-based ligands (H2L1–6) with assorted functional groups appended to them have been used for the synthesis of dicopper(II) complexes 1–6 having a Cu(μ-OH)Cu core. The crystal structures of 1–6 show that while every Cu(II) ion is ligated within the N3 pincer cavity of a potentially multidentate ligand, two Cu(II) centers are bridged by a hydroxide group. Notably, the Cu(μ-OH)Cu core is encased within the secondary coordination sphere intricately created by the appended groups. While complexes 1 and 2 exhibit the presence of an H-bond acceptor in the proximity of the Cu(μ-OH)Cu core, complexes 3 and 4 display the occurrence of both the H-bond donor as well as H-bond acceptor groups in the vicinity of the Cu(μ-OH)Cu core. In contrast, complexes 5 and 6 present modified secondary coordination spheres around the Cu(μ-OH)Cu core with limited H-bonding interacting groups in 5 and no such groups in 6. We show that the extent of H-bonding by the appended groups modulates not only the Cu–OH bond distance, Cu(μ-OH)Cu angle and Cu–Cu separation but also the Cu2+/Cu+ redox potential. All six complexes were utilized for their ability to oxidize 3,5-di-tert-butylcatechol, and the catecholase activity results have been correlated to the secondary coordination sphere created by the appended groups in all six complexes.


 Please wait while we load your content...
Please wait while we load your content...