Planar-chiral ferrocenylphosphine-borane complexes featuring agostic-type B–H⋯E (E = Hg, Sn) interactions†
Abstract
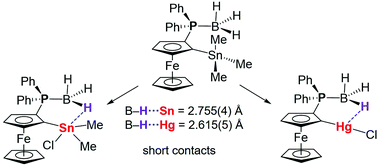
The synthesis of ferrocenylphosphine-borane adducts 1,2-fc(E)(PPh2·BH3) (E = SnR2R′, HgX; 1,2-fc = 1,2-ferrocenediyl) that are substituted with organotin or organomercury Lewis acid moieties in ortho-position is presented. Several compounds that feature two ferrocenylphosphine-borane moieties bridged by Sn or Hg are also introduced. The products are fully characterized by multinuclear NMR spectroscopy, high-resolution MALDI-TOF mass spectrometry and elemental analysis. The attachment of the Lewis acid substituent to the same Cp ring of the ferrocene results in planar-chirality and the close proximity between the boron hydride group and the Lewis acid is expected to allow for agostic-type B–H⋯E (E = Sn, Hg) interactions. Structural investigations by X-ray diffraction reveal a short B–H⋯Sn contact of 2.755(4) Å for 1,2-fc(SnMe2Cl)(PPh2·BH3), which is only the second reported example of such a short agostic-type contact involving a coordinatively saturated tin(IV) center. In contrast, for the tetraorganotin derivatives 1,2-fc(SnMe3)(PPh2·BH3) and [1,2-fc(PPh2)](μ-SnMe2)[1,2-fc(PPh2·BH3)], in which the Lewis acidity of the tin atom is weaker than in 1,2-fc(SnMe2Cl)(PPh2·BH3), the B–H⋯Sn distances are much longer but still within the sum of the van der Waals radii of Sn and H (∑vdW = 3.27 Å). The chloromercury-substituted ferrocenylphosphine-borane 1,2-fc(HgCl)(PPh2·BH3) shows a similarly short B–H⋯Hg contact of 2.615(5) Å (∑vdW = 3.15 Å). Inspection of the extended structure of 1,2-fc(HgCl)(PPh2·BH3) reveals that the Lewis acidic mercury atom is also involved in intermolecular Hg⋯Cl interactions with a neighboring molecule. An analysis of 31P and 11B NMR data reveals a correlation between the chemical shifts and the Lewis acidity of the adjacent organotin/mercury substituent. Structure optimization of 1,2-fc(SnMe3)(PPh2·BH3) and 1,2-fc(SnMe2Cl)(PPh2·BH3) by density functional theory (DFT) indicates B–H⋯Sn contacts respectively of 3.129 and 2.631 Å that are close to the experimental values. Natural bond orbitals (NBO) and atom in molecules (AIM) analyses reveals a B–H→Sn donor–acceptor interaction energy of 5.46 kcal mol−1 and a B–H⋯Sn bond path for the chlorodimethyltin-substituted derivative with a modest electron density ρ(r) of 0.0082 a.u. and a positive Laplacian at the bond critical point.


 Please wait while we load your content...
Please wait while we load your content...