Circularly polarized luminescence on dinuclear Tb(iii) and Eu(iii) complexes with (S-) and (R-) 2-phenylpropionate†
Abstract
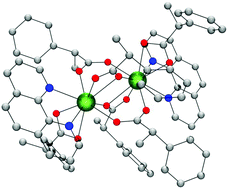
The reaction of Ln(NO3)2·6H2O (Ln = Tb and Eu) with (S)-(+)-2-phenylpropionic acid (S-HL) and 1,10-phenanthroline (phen) in EtOH/H2O allows the isolation of the dinuclear chiral compounds of the formula [Ln2(S-L)6(phen)2]·2.5·S-HL in which Ln = Tb (S-1), Ln = Eu (S-2). The same synthesis by using (R)-(−)-2-phenylpropionic acid (R-HL) instead of (S)-(+)-2-phenylpropionic acid allows the isolation of the enantiomeric compounds with the formula [Ln2(R-L)6(phen)2]·2.5·R-HL where Ln = Tb (R-1), Ln = Eu (R-2). All compounds show sensitized luminescence. The luminescence study, including the circularly polarized luminescence spectra of the four compounds, is reported. The magnetic behavior of S-1 and S-2 is also reported.


 Please wait while we load your content...
Please wait while we load your content...