Cytotoxic gold(iii) complexes incorporating a 2,2′:6′,2′′-terpyridine ligand framework – the impact of the substituent in the 4′-position of a terpy ring†
Abstract
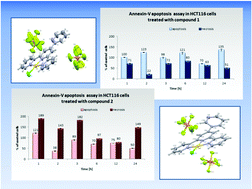
Two gold(III) complexes incorporating 2,2′:6′,2′′-terpyridine derivatives have been synthesised and characterized, and the possibility of tuning the cytotoxic activity by structural modifications of a terpy ligand has been examined. Both complexes [AuCl(4′-R1-terpy)](PF6)2 (1) and [AuCl(4′-R2-terpy)](PF6)2 (2), where R1 is 2-pyridyl and R2 is 3-pyridyl, show good anti-proliferative activities against HCT116, which are higher in relation to those of the free ligands, [AuCl(terpy)](PF6)2 and standard anticancer drug cisplatin. The cytotoxic properties of the gold complexes were examined by MTS assay, cell cycle and apoptosis analysis, ROS measurements, determination of mitochondrial membrane potential and mass, and staining of phosphatidylserine with Annexin-V antibody FITC-conjugated together with PI.


 Please wait while we load your content...
Please wait while we load your content...