Sterically directed nitronate complexes of 2,6-di-tert-butyl-4-nitrophenoxide with Cu(ii) and Zn(ii) and their H-atom transfer reactivity†
Abstract
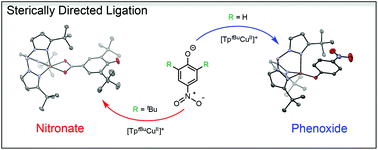
The bulky 2,6-di-tert-butyl-4-nitrophenolate ligand forms complexes with [TptBuCuII]+ and [TptBuZnII]+ binding via the nitro group in an unusual nitronato-quinone resonance form (TptBu = hydro-tris(3-tert-butyl-pyrazol-1-yl)borate). The Cu complex in the solid state has a five-coordinate κ2-nitronate structure, while the Zn analogue has a four-coordinate κ1-nitronate ligand. 4-Nitrophenol, without the 2,6-di-tert-butyl substituents, instead binds to [TptBuCuII]+ through the phenolate oxygen. This difference in binding is very likely due to the steric difficulty in binding a 2,6-di-tert-butyl-phenolate ligand to the [TptBuMII]+ unit. TptBuCuII(κ2-O2NtBu2C6H2O) reacts with the hydroxylamine TEMPO–H (2,2,6,6-tetramethylpiperidin-1-ol) by abstracting a hydrogen atom. This system thus shows an unusual sterically enforced transition metal–ligand binding motif and a copper–phenolate interaction that differs from what is typically observed in biological and chemical catalysis.


 Please wait while we load your content...
Please wait while we load your content...