Synthesis and characterization of a series of nickel(ii) alkoxide precursors and their utility for Ni(0) nanoparticle production†
Abstract
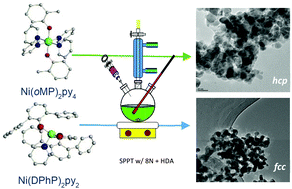
A series of nickel(II) aryloxide ([Ni(OAr)2(py)x]) precursors was synthesized from an amide-alcohol exchange using [Ni(NR2)2] in the presence of pyridine (py). The H-OAr selected were the mono- and di-ortho-substituted 2-alkyl phenols: alkyl = methyl (H-oMP), iso-propyl (H-oPP), tert-butyl (H-oBP) and 2,6-di-alkyl phenols (alkyl = di-iso-propyl (H-DIP), di-tert-butyl (H-DBP), di-phenyl (H-DPhP)). The crystalline products were solved as solvated monomers and structurally characterized as [Ni(OAr)2(py)x], where x = 4: OAr = oMP (1), oPP (2); x = 3: OAr = oBP (3), DIP (4); x = 2: OAr = DBP (5), DPhP (6). The excited states (singlet or triplet) and various geometries of 1–6 were identified by experimental UV-vis and verified by computational modeling. Magnetic susceptibility of the representative compound 4 was fit to a Curie Weiss model that yielded a magnetic moment of 4.38(3)μB consistent with a Ni2+ center. Compounds 1 and 6 were selected for decomposition studied under solution precipitation routes since they represent the two extremes of coordination. The particle size and crystalline structure were characterized using transmission electron microscopy (TEM) and powder X-ray diffraction (PXRD). The materials isolated from 1 and 6 were found by TEM to form irregular shape nanomaterials (8–15 nm), which by PXRD were found to be Ni0 hcp (PDF: 01-089-7129) and fcc (PDF: 01-070-0989), respectively.


 Please wait while we load your content...
Please wait while we load your content...