A new adsorbent of a Ce ion-implanted metal–organic framework (MIL-96) with high-efficiency Ce utilization for removing fluoride from water†
Abstract
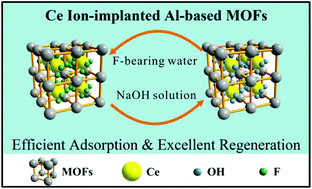
A novel Ce(III) ion-implanted aluminum-trimesic metal–organic framework (Ce–MIL-96) was synthesized for the first time via alcohol-solvent incipient wetness impregnation. Compared to previously reported Ce-contained adsorbents, the fluoride adsorption performance of the new ion-implanted metal–organic framework demonstrated much higher adsorption capacity and more efficient regeneration of Ce. In a wide pH range of 3 to 10, Ce–MIL-96 maintained constant adsorption performance for fluoride, and the residual Ce and Al in the treated solution were below the safe limits in drinking water. The maximum adsorption capacity of Ce–MIL-96 was 38.65 mg g−1 at 298 K. Excluding the contribution of MIL-96, the maximum adsorption capacity of Ce ions was 269.75 mg g−1, which demonstrated that the service efficiency of cerium in Ce–MIL-96 is about 6 times that in Ce2O3, nearly 10 times that in Ce-mZrp, and double that in Mn–Ce oxides. There was no significant influence on fluoride removal by Ce–MIL-96 due to the presence of chloride, nitrate, sulfate, bicarbonate or phosphate. Moreover, the adsorption capacity of Ce–MIL-96 remained at more than 70% after nine cycles of adsorption–desorption. Due to this excellent adsorption performance and its regeneration properties, Ce–MIL-96 is a promising adsorbent for the removal of fluoride from groundwater.


 Please wait while we load your content...
Please wait while we load your content...