Sb-Based antiferromagnetic oxychlorides: MSb2O3(OH)Cl (M = Mn, Fe, Co) with 2D spin-dimer structures†
Abstract
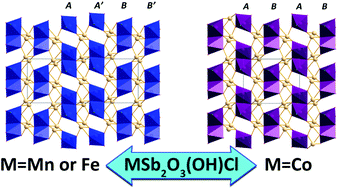
Three new Sb-based antiferromagnetic oxychlorides: MnSb2O3(OH)Cl (1), FeSb2O3(OH)Cl (2), and CoSb2O3(OH)Cl (3) have been synthesized by using hydrothermal reaction methods. Their crystal structures are isostructural and incorporate two types of structural units: sphenoid SbO4 polyhedra and M2O8Cl2 (M = Mn, Fe, Co) dimers, that share edges forming a two dimensional (2D) MSb2O3(OH)Cl network. The neutral MSb2O3(OH)Cl 2D layers are arranged from ABAB-type for compounds 1 and 2 to AA′B′B-type for 3 with smaller cation radii. Magnetic measurements show that 1 belongs to an antiferromagnetic material, while 2 and 3 have weak ferromagnetic properties with unsaturated ferromagnetism originating from spin-canting below the transition temperature. In addition, the optical absorption band gaps were determined to be 3.8, 2.4, and 3.6 eV for 1, 2, and 3, respectively. This work demonstrates that utilizing both halide ions and lone-pair elements simultaneously in one structure is a feasible and practical route to reduce framework connectivity in order to synthesize new low-dimensional oxyhalides.

 Please wait while we load your content...
Please wait while we load your content...