One-pot synthesis of folic acid encapsulated upconversion nanoscale metal organic frameworks for targeting, imaging and pH responsive drug release†
Abstract
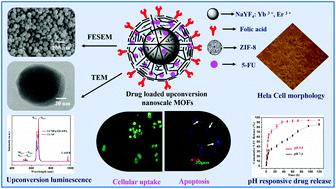
In this work, a new theranostic nanoplatform is developed to construct an anticancer drug carrier by integrating the distinct advantages of upconversion nanoparticles (UCNPs) and metal organic frameworks (MOFs) encapsulated with a targeting ligand. Here NaYF4:Yb3+,Er3+ is chosen as an upconversion nanoparticle for its high luminescence properties. Then, folic acid encapsulated Zeolitic Imidazolate Framework-8 (ZIF-8) is directly coated on UCNPs in one step to form a monodispersed core–shell structured nanocomposite (labeled as UCNPs@ZIF-8/FA). The synthesized upconversion nanoscale MOFs (NMOFs) are simultaneously used as a targeted anticancer drug carrier and in cellular imaging. The UCNP@ZIF-8/FA nanocomposites are found to be nontoxic towards the human cervix adenocarcinoma (HeLa) and mouse fibroblast (L929) cell lines via a cell viability assay. It is worthwhile noting that, the anticancer drug 5-fluorouracil (5-FU) is absorbed into UCNP@ZIF-8/FA nanocomposites (loading amount 685 mg g−1) and also pH responsive drug release is observed. The as-prepared 5-FU loaded UCNP@ZIF-8/FA nanocomposites exhibited greater cytotoxicity towards HeLa cells due to the folate receptor-mediated endocytosis. Our study highlights the potential of developing multifunctional upconversion NMOFs for simultaneous targeted cellular imaging with delivery of anticancer drugs.

 Please wait while we load your content...
Please wait while we load your content...