First quadruple-glycine bridging mono-lanthanide-substituted borotungstate hybrids†
Abstract
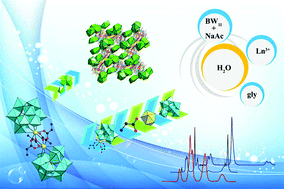
A class of novel organic–inorganic hybrid lanthanide (Ln)-substituted Keggin-type borotungstates K4Na4H4[Ln2(gly)4(α-BW11O39)2]·23H2O [Ln = Ce3+ (1), Pr3+ (2), Nd3+ (3), Sm3+ (4), Eu3+ (5), Tm3+ (6); gly = glycine] have been synthesized from the reaction of K8[BW11O39H]·13H2O, NaAc·6H2O and Ln(NO3)3·6H2O by employing gly ligands as structure-stabilizing agents in the conventional aqueous solution system and structurally characterized by elemental analyses, IR spectroscopy, thermogravimetric (TG) analyses, powder X-ray diffraction (PXRD) and single-crystal X-ray diffraction. The common prominent structural feature of isomorphic 1–6 is that all of them consist of two mono-Ln-substituted Keggin [Ln(α-BW11O39)]6− fragments linked by four gly ligands, furnishing an intriguing dimeric assembly of the quadruple-gly-connective mono-Ln-substituted borotungstate, in which each carboxylic oxygen atom from gly ligands is bound to Ln3+ cations in the μ2-O or μ3-O mode. To the best of our knowledge, 1–6 represent the first examples of inorganic–organic hybrid Ln-substituted borotungstates functionalized by quadruple amino acid bridges. The solid-state photoluminescence properties of 3–5 have been determined at ambient temperature and the photoluminescence emission spectra exhibit the characteristic emission bands derived from Ln3+ centers. The thermostability of 1–6 has been studied and the thermal decomposition procedure of 3 has been comprehensively investigated with the assistance of variable-temperature PXRD patterns and variable-temperature IR spectra. Furthermore, magnetic susceptibility measurements of 1, 2 and 4 have been conducted.

 Please wait while we load your content...
Please wait while we load your content...