N–H⋯O and N–H⋯Cl supported 1D chains of heterobimetallic CuII/NiII–SnIV cocrystals†
Abstract
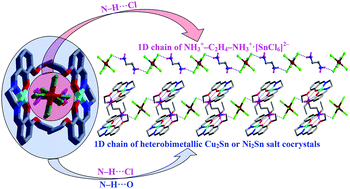
The Schiff base H2L1 [N,N′-ethylenebis(3-methoxysalicylaldimine)] or H2L2 [N,N′-ethylenebis(3-ethoxysalicylaldimine)] was reacted with MCl2·xH2O and SnCl4·5H2O to afford the supramolecular heterobimetallic systems (H2ED)2+·2[ML]·[SnCl6]2− [M = Cu, L = L1 (1), L = L2 (2); M = Ni, L = L1 (3), L = L2 (4); ED = 1,2-ethylenediamine], whose structures were established by single crystal X-ray analyses. Each structure includes different entities, viz. a mononuclear [CuL]/[NiL] neutral complex (coformer), a hexachlorostannate dianion [SnCl6]2−, a 1,2-ethylenediammonium dication (H2ED2+) and, only in 2 and 4, a methanol molecule. Based on the work of Grothe et al. (Cryst. Growth Des., 2016, 16, 3237–3243), compounds 1 and 3 are cocrystal salts, 2 and 4 are cocrystal salt solvates. The ionic pairs (H2ED)2+·[SnCl6]2− in 1–4 are encapsulated by the Cu- or Ni-complexes, and stabilized by N–H⋯O and one N–H⋯Cl bond interactions leading to infinite 1D chains. The antimicrobial studies of 1–4 against yeasts (C. albicans and S. cerevisiae) and Gram-positive (S. aureus and E. faecalis) and -negative bacteria (P. aeruginosa and E. coli) indicate that the Ni2Sn systems (3 and 4) are more active than the analogous Cu2Sn ones (1 and 2).
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...