Nickel-catalyzed transfer hydrogenation of ketones using ethanol as a solvent and a hydrogen donor†
Abstract
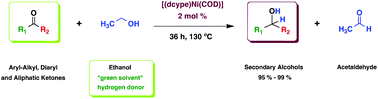
We report a nickel(0)-catalyzed direct transfer hydrogenation (TH) of a variety of alkyl–aryl, diaryl, and aliphatic ketones with ethanol. This protocol implies a reaction in which a primary alcohol serves as a hydrogen atom source and solvent in a one-pot reaction without any added base. The catalytic activity of the nickel complex [(dcype)Ni(COD)] (e) (dcype: 1,2-bis(dicyclohexyl-phosphine)ethane, COD: 1,5-cyclooctadiene), towards transfer hydrogenation (TH) of carbonyl compounds using ethanol as the hydrogen donor was assessed using a broad scope of ketones, giving excellent results (up to 99% yield) compared to other homogeneous phosphine–nickel catalysts. Control experiments and a mercury poisoning experiment support a homogeneous catalytic system; the yield of the secondary alcohols formed in the TH reaction was monitored by gas chromatography (GC) and NMR spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...