The effect of counteranions on the molecular structures of phosphanegold(i) cluster cations formed by polyoxometalate (POM)-mediated clusterization†
Abstract
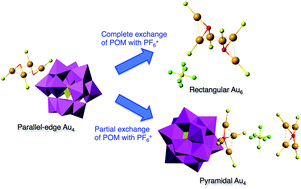
The effect of counteranions on the molecular structures of phosphanegold(I) cluster cations formed by polyoxometalate (POM)-mediated clusterization was investigated. A novel intercluster compound, [{(AuLCl)2(μ-OH)}2]3[α-PMo12O40]2·3EtOH (1-PMo12), was obtained as orange-yellow plate crystals in 12.0% yield from a 6 : 1 molar ratio reaction of the monomeric phosphanegold(I) carboxylato complex [Au(RS-pyrrld)(LCl)] (RS-Hpyrrld = RS-2-pyrrolidone-5-carboxylic acid; LCl = tris(4-chlorophenyl)phosphane) in CH2Cl2 with the free acid-form of Keggin polyoxometalate (POM), H3[α-PMo12O40]·14H2O. An EtOH/H2O (5 : 1, v/v) solvent mixture was used. The dimeric cation [{(AuLCl)2(μ-OH)}2]2+ in 1-PMo12 was in a parallel-edge arrangement that was formed by self-assembly through the inter-cationic aurophilic interactions of the μ-OH-bridged dinuclear phosphanegold(I) cation. The POM anion in 1-PMo12 was successfully exchanged with a smaller PF6− anion by the use of an anion-exchange resin. POM-free, colorless block crystals of [{(AuLCl)3(μ3-O)}2](PF6)2·4CH2Cl2 (2-PF6) were obtained by vapor diffusion in 14.1% yield. During the synthesis of 2-PF6, a compound with mixed counteranions (one POM and one PF6− anion), i.e. [{(AuLCl)4(μ4-O)}]2[α-PMo12O40]PF6 (3-PMo12PF6), was obtained in 66.4% yield. Both products were characterized by elemental analysis, TG/DTA, FT-IR, 31P{1H} NMR, 1H NMR, and X-ray crystallography. X-ray crystallography revealed that the countercation in 2-PF6 was the dimeric cation of the μ3-O-bridged tris{phosphanegold(I)} species, whereas that in 3-PMo12PF6 consisted of an unusual μ4-O-bridged tetragonal-pyramidal tetrakis{phosphanegold(I)} cation. Therefore, we concluded that the POM anion significantly contributed to the stabilization of these countercations (parallel-edged arrangement in 1-PMo12 and μ4-O-bridged tetragonal-pyramid in 3-PMo12PF6). Moreover, the previously reported yellow crystals of [{(AuLF)2(μ-OH)}2]3[PMo12O40]2·3EtOH (4-PMo12: LF = tris(4-fluoro phenyl)phosphane) were successfully converted to the POM-free crystalline OTf− salt [{(AuLF)2(μ-OH)}2](OTf)2·0.5Et2O (4-OTf) by the use of an anion-exchange resin. X-ray crystallography also revealed that the parallel-edge arrangement of the dimeric cation in 4-PMo12 was converted to the crossed-edge arrangement of that in 4-OTf. These results illustrate that the Au⋯OPOM and hydrogen-bonding (C–H⋯OPOM and O–H⋯OPOM) interactions between the phosphanegold(I) cluster cation and the Keggin POM anion in the solid state significantly contribute to the structure, composition, and stability of the phosphane gold(I) cluster cations in 4-PMo12.

 Please wait while we load your content...
Please wait while we load your content...