Ruthenium(ii) complexes of hemilabile pincer ligands: synthesis and catalysing the transfer hydrogenation of ketones†
Abstract
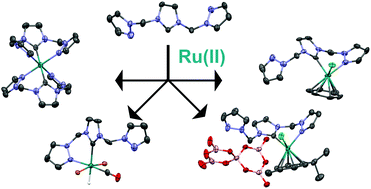
A series of Ru(II) complexes were synthesised based on a hemilabile pyrazole-N-heterocyclic carbene (NHC)-pyrazole (C3N2H3)CH2(C3N2H2)CH2(C3N2H3) NCN pincer ligand 1. All complexes were fully characterised using single crystal X-ray crystallography and multinuclear NMR spectroscopy. Hemilabile ligands provide flexible coordination modes for the coordinating metal ion which can play a significant effect on the efficiency and mechanism of catalysis by the resulting complex. Here we observed and isolated mono-, bi- and tri-dentate complexes of both Ag(I) and Ru(II) with 1 in which the resultant coordination mode was controlled by careful reagent selection. The catalytic activity of the Ru(II) complexes for the transfer hydrogenation reaction of acetophenone with isopropanol was investigated. The unexpected formation of the pentaborate anion, [B5O6(OH)4]−, during the synthesis of complex 6a was found to have an unexpected positive effect by enhancing the catalysis rate. This work provides insights into the roles that different coordination modes, counterions and ligand hemilability play on the catalytic activity in transfer hydrogenations.


 Please wait while we load your content...
Please wait while we load your content...