Increasing the triplet lifetime and extending the ground-state absorption of biscyclometalated Ir(iii) complexes for reverse saturable absorption and photodynamic therapy applications†
Abstract
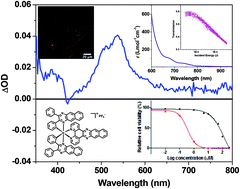
The synthesis, photophysics, reverse saturable absorption, and photodynamic therapeutic effect of six cationic biscyclometalated Ir(III) complexes (1–6) with extended π-conjugation on the diimine ligand and/or the cyclometalating ligands are reported in this paper. All complexes possess ligand-localized 1π,π* absorption bands below 400 nm and charge-transfer absorption bands above 400 nm. They are all emissive in the 500–800 nm range in deoxygenated solutions at room temperature. All complexes exhibit strong and broad triplet excited-state absorption at 430–800 nm, and thus strong reverse saturable absorption for ns laser pulses at 532 nm. Complexes 1–4 are strong reverse saturable absorbers at 532 nm, while complex 6 could be a good candidate as a broadband reverse saturable absorber at 500–850 nm. The degree of π-conjugation of the diimine ligand mainly influences the 1π,π* transitions in their UV-vis absorption spectra, while the degree of π-conjugation of the cyclometalating ligand primarily affects the nature and energies of the lowest singlet and emitting triplet excited states. However, the lowest-energy triplet excited states for complexes 3–6 that contain the same benzo[i]dipyrido[3,2-a:2′,3′-c]phenazine (dppn) diimine ligand but different cyclometalating ligands remain the same as the dppn ligand-localized 3π,π* state, which gives rise to the long-lived, strong excited-state absorption in the visible to the near-IR region. All of the complexes exhibit a photodynamic therapeutic effect upon visible or red light activation, with complex 6 possessing the largest phototherapeutic index reported to date (>400) for an Ir(III) complex. Interactions with biological targets such as DNA suggest that a novel mechanism of action may be at play for the photosensitizing effect. These Ir(III) complexes also produce strong intracellular luminescence that highlights their potential as theranostic agents.

 Please wait while we load your content...
Please wait while we load your content...