Optimum bifunctionality in a 2-(2-pyridyl-2-ol)-1,10-phenanthroline based ruthenium complex for transfer hydrogenation of ketones and nitriles: impact of the number of 2-hydroxypyridine fragments†
Abstract
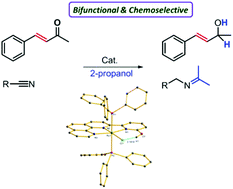
Considerable differences in reactivity and selectivity for 2-hydroxypyridine (2-HP) derived ruthenium complexes in transfer hydrogenation are described. Bifunctional Ru(II)-(phenpy-OH) [phenpy-OH: 2-(2-pyridyl-2-ol)-1,10-phenanthroline] complex (2) exhibited excellent catalytic activity in transfer hydrogenation (TH) of ketones and nitriles. Notably, in comparison with all the reported 2-hydroxypyridine (2-HP) derived ruthenium complexes in transfer hydrogenation, complex 2 displayed significantly higher activity. Additionally, exploiting the metal–ligand cooperativity in complex 2, chemoselective TH of ketones was achieved and sterically demanding ketones were readily reduced. An outer-sphere mechanism is proposed for this system as exogenous PPh3 has no significant effect on the rate of this reaction. This is a rare example of a highly active bifunctional Ru(II) catalyst bearing only one 2-HP unit.

 Please wait while we load your content...
Please wait while we load your content...