Fluorescent LaVO4:Eu3+ micro/nanocrystals: pH-tuned shape and phase evolution and investigation of the mechanism of detection of Fe3+ ions†
Abstract
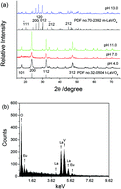
LaVO4:Eu3+ micro/nanocrystals with various shapes were hydrothermally synthesized by adjusting the pH of the system at 180 °C for 12 h in the presence of ethylenediaminetetraacetic acid (EDTA). The shape and phase of the final product were characterized by field emission scanning electron microscopy (FESEM) and X-ray powder diffraction (XRD). Experiments showed that when the other conditions were kept unchanged, the shape of the final product changed from hollow microspheres constructed by nanorods to long nanorods, to short nanorods and finally to grains with microscale sizes with the pH increase from 4.0, 7.0, 11.0 to 13.0 in the system. Meanwhile, the t-LaVO4 phase was always obtained from the system at pH below 13.0 and the m-LaVO4 phase was formed at pH 13.0. It was found that the final product with various shapes presented different luminescence performances. LaVO4:Eu3+ nanorods obtained from the system at pH 11.0 displayed the strongest luminescence and good fluorescence stability in water. Also, the above strong PL spectrum could be quenched by Fe3+ ions without the interference of other ions, indicating that the present product could be used as an efficient fluorescent probe for highly selective detection of Fe3+ ions in water systems. The fluorescence quenching mechanism was investigated simultaneously.

 Please wait while we load your content...
Please wait while we load your content...