Selone-stabilized aryltellurenyl cations†
Abstract
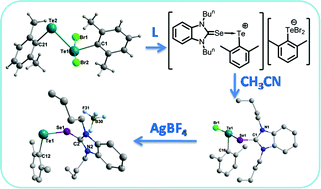
Controlled bromination of a diarylditelluride, R2Te2 (R = 2,6-dimethylphenyl) (6) in dichloromethane led to the formation of a TeII–TeIV mixed-valent tellurenyl bromide, RBr2TeTeR (7). A further reaction of 7 with 1,3-dibutylbenzimidazolin-2-selone, C15H22N2Se (L) (9), produced the first selone adduct of the 2,6-dimethylphenyltellurenyl cation with the 2,6-dimethylphenyltellurium dibromide anion, [(2,6-Me2C6H3)Te(L)]+[(2,6-Me2C6H3)TeBr2]− (10). The red colored cationic adduct 10 is not stable in acetonitrile and disproportionated to give the selone adduct of 2,6-dimethylphenyltellurenyl bromide, [(2,6-Me2C6H3)Te(L)Br] (11b) and bis(2,6-dimethylphenyl)tellurium dibromide, [(2,6-Me2C6H3)2TeBr2], (13). The metathesis reaction of 11b with AgBF4 produced a stable dark red colored selone adduct of the 2,6-dimethylphenyltellurenyl cation with the BF4− anion, [(2,6-Me2C6H3)Te(L)]+BF4− (15). The selone adducts of aryltellurenyl halides, i.e. [(2,6-Me2C6H3)Te(L)X] (X = Cl, Br, I) (11a–11c), have been synthesized by a one-pot reaction of 6 with an equimolar mixture of 9 and 1,3-dibutylbenzimidazolin-2-dihaloselones, C15H22N2SeX2 (14a–14c). Triphenylphosphine (PPh3), when treated with [(2,6-Me2C6H3)Te(L)X] (11a–11c), substitutes selone from the adduct to afford the triphenylphosphine adducts of aryltellurenyl halides, [(2,6-Me2C6H3)Te(PPh3)X] (16a–16c).
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...