Structurally versatile phosphine and amine donors constructed from N-heterocyclic olefin units†
Abstract
A general strategy for the synthesis of hindered N- and P-based donors is presented whereby the strongly electron releasing N-heterocyclic olefin (NHO) unit, IPr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, (IPr
CH–, (IPr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– = [(HCNDipp)2C
CH– = [(HCNDipp)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH]−; Dipp = 3,6-iPr2C6H2) is linked to terminally bound phosphine and amine donors. Preliminary coordination chemistry is presented involving phosphine (IPr
CH]−; Dipp = 3,6-iPr2C6H2) is linked to terminally bound phosphine and amine donors. Preliminary coordination chemistry is presented involving phosphine (IPr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)PR2 (R = iPr and Ph) and amine (IPr
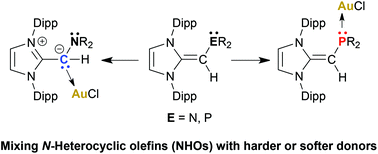
CH)PR2 (R = iPr and Ph) and amine (IPr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)NMe2 ligands and the Lewis acids BH3 and AuCl. Interestingly, (IPr
CH)NMe2 ligands and the Lewis acids BH3 and AuCl. Interestingly, (IPr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)NMe2 binds AuCl through an exocyclic olefin unit, while the softer phosphorus centers in (IPr
CH)NMe2 binds AuCl through an exocyclic olefin unit, while the softer phosphorus centers in (IPr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)PR2 coordinate to yield Au–P linkages; thus the reported NHO-based ligands exhibit tunable binding modes to metals.
CH)PR2 coordinate to yield Au–P linkages; thus the reported NHO-based ligands exhibit tunable binding modes to metals.
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...