Variable coordination modes and catalytic dehydrogenation of B-phenyl amine–boranes†
Abstract
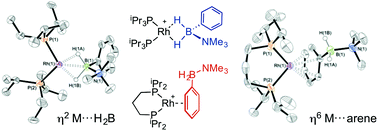
The chemistry of N-substituted amine–boranes and their reactivity towards transition metal centres is well established but the chemistry of B-substituted amine–boranes is not. Here we present the coordination chemistry of H2PhB·NMe3 towards a range of Rh(I) fragments with different P–Rh–P ligand bite angles, {Rh(PiPr3)2}+, {Rh(PiBu3)2}+, {Rh(iPr2P(CH2)3PiPr2)}+, {Rh(Ph2P(CH2)nPPh2)}+ (n = 3, 5), as characterised by NMR spectroscopy and single-crystal X-ray diffraction. This reveals a difference in the coordination mode of the amine–borane, with large bite angle fragments favouring η2-coordination through a sigma-interaction with BH2, whereas fragments with small bite angles favour η6-coordination through the aryl group of the amine–borane. The catalytic dehydrocoupling of H2PhB·NMe2H is also explored, with the aminoborane HPhB![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe2 found to be the sole dehydrogenation product. Stoichiometric reactivity with H2PhB·NMe2H again showed small bite angle fragments to prefer η6-aryl coordination, while the larger bite angle {Rh(PiPr3)2}+ gave rapid dehydrogenation to form a mixture of the Rh(III) dihydride [Rh(PiPr3)2(H)2(η2-H2PhB·NMe2H)][BArF4] and the low coordinate aminoboryl complex [Rh(PiPr3)2(H)(BPhNMe2)][BArF4]. These results suggest that precatalysts which η6-bind arenes strongly should be avoided for the dehydrocoupling of amine–boranes bearing aryl substituents.
NMe2 found to be the sole dehydrogenation product. Stoichiometric reactivity with H2PhB·NMe2H again showed small bite angle fragments to prefer η6-aryl coordination, while the larger bite angle {Rh(PiPr3)2}+ gave rapid dehydrogenation to form a mixture of the Rh(III) dihydride [Rh(PiPr3)2(H)2(η2-H2PhB·NMe2H)][BArF4] and the low coordinate aminoboryl complex [Rh(PiPr3)2(H)(BPhNMe2)][BArF4]. These results suggest that precatalysts which η6-bind arenes strongly should be avoided for the dehydrocoupling of amine–boranes bearing aryl substituents.
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...