Enhanced hydrogen storage properties of the 2LiBH4–MgH2 composite with BaTiO3 as an additive†
Abstract
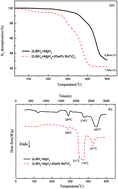
The 2LiBH4–MgH2 + 20 wt% BaTiO3 composite was prepared by ball-milling LiBH4, MgH2 and BaTiO3, and the effect of BaTiO3 on the hydrogen storage properties of the composite was investigated. TG-DSC results show that the onset dehydrogenation temperature of the composite is 299 °C, which is 124 °C lower than that of 2LiBH4–MgH2, and the dehydrogenation amount of the composite increases from 6.86 wt% to 7.48 wt% at 500 °C. Kinetic tests show that the dehydrogenation amount of 2LiBH4–MgH2 + 20 wt% BaTiO3 reaches 1.5 wt% within 400 seconds, almost 10 times that of 2LiBH4–MgH2. BaTiO3 reacts with LiBH4 during the dehydrogenation of the composite and generates BaB6 and TiO2. BaB6 is beneficial to lower the stability of LiBH4, while TiO2 has a catalytic effect in improving the hydrogenation/dehydrogenation kinetics of the reaction between Mg and LiBH4.


 Please wait while we load your content...
Please wait while we load your content...