Understanding the contribution of hydroxyl to the energy band of a semiconductor: Bi2O(OH)2SO4vs. Bi6S2O15†
Abstract
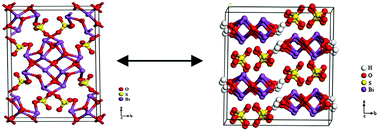
It is still a big challenge to facilely tune the energy bands of a semiconductor. Herein, we have mainly investigated energy bands and photochemical properties of Bi6S2O15 and Bi2O(OH)2SO4, which have very similar layered structures. It is found that the hydroxyls have down shifted the conduction band (CB, 0.21 eV) and valence band (VB, 4.39 eV) of Bi2O(OH)2SO4, compared with those (CB = 0 eV; VB = 3.36 eV) of Bi6S2O15. Moreover, the main oxidative species of Bi6S2O5 and Bi2O(OH)2SO4 are holes (h+) and hydroxyl radicals (˙OH) for the degradation of rhodamine B (RhB) dye, respectively. This obvious difference has been mainly attributed to the hydroxyls, which have changed the energy band structure and the band gap. In addition, we have also investigated the morphology-dependent properties of Bi2O(OH)2SO4. Under ultraviolet light irradiation (λ ≤ 420 nm), Bi2O(OH)2SO4 microspheres show an activity 1.3 times and 2.2 times higher than the long flakes and straw sheaves for the degradation of (RhB), respectively. This study provides us a new idea that we can facilely tune the energy band of a semiconductor by introducing or removing hydroxyl or other anions.

 Please wait while we load your content...
Please wait while we load your content...