Cationic ruthenium alkylidene catalysts bearing phosphine ligands†
Abstract
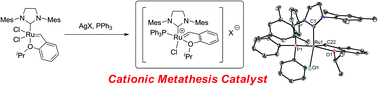
The discovery of highly active catalysts and the success of ionic liquid immobilized systems have accelerated attention to a new class of cationic metathesis catalysts. We herein report the facile syntheses of cationic ruthenium catalysts bearing bulky phosphine ligands. Simple ligand exchange using silver(I) salts of non-coordinating or weakly coordinating anions provided either PPh3 or chelating Ph2P(CH2)nPPh2 (n = 2 or 3) ligated cationic catalysts. The structures of these newly reported catalysts feature unique geometries caused by ligation of the bulky phosphine ligands. Their activities and selectivities in standard metathesis reactions were also investigated. These cationic ruthenium alkylidene catalysts reported here showed moderate activity and very similar stereoselectivity when compared to the second generation ruthenium dichloride catalyst in ring-closing metathesis, cross metathesis, and ring-opening metathesis polymerization assays.

 Please wait while we load your content...
Please wait while we load your content...