Postcomplexation synthetic routes to dipyrrin complexes†
Abstract
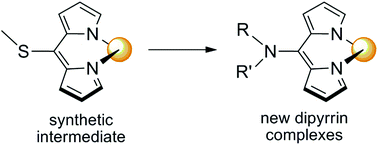
We report a postfunctionalization synthetic route to dipyrrin complexes that gives access to a broad range of new complexes. This route involves the coordination of a 5-methylthiodipyrrinato ligand to a metal centre followed by displacement of the thiomethyl moiety by a nucleophile. Using rhenium(I) as a platform and amine nucleophiles, we show how complexes that would be difficult or impossible to synthesize via traditional methods can now be accessed.

 Please wait while we load your content...
Please wait while we load your content...