Lanthanide salen-type complexes exhibiting single ion magnet and photoluminescent properties†
Abstract
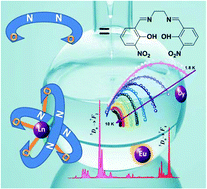
Salen-type mononuclear lanthanide complexes with formula (Et3NH)[Ln(3-NO2-salen)2]·solvent (Ln = Eu (1Eu), Tb (2Tb), Dy (3Dy), Ho (4Ho), Er (5Er), Yb (6Yb); 3-NO2-salen2− = N,N′-bis(3-nitro-salicylaldehyde)ethylenediamine dianion) are reported. These compounds are isostructural in which two crystallographically distinct 3-NO2-salen2− act as tetradentate ligands encapsulating the lanthanide ions in a meridional mode forming the [LnN4O4] cores. Slow magnetization relaxation processes associated with single ion magnet (SIM) behaviors are observed in complexes 3Dy, 5Er and 6Yb with the Kramer ions but not in 2Tb and 4Ho with non-Kramer ions. Photoluminescence studies reveal that complexes 1Eu, 5Er and 6Yb exhibit characteristic lanthanide luminescence with sharp and well-separated emission bands. Complex 1Eu is of particular interest in which the organic ligand functioning as a powerful absorbing sensitizer apparently broadens the excitation range into 300–500 nm with the maximum of 460 nm.


 Please wait while we load your content...
Please wait while we load your content...