Carbene insertion into a P–H bond: parent phosphinidene–carbene adducts from PH3 and bis(phosphinidene)mercury complexes†
Abstract
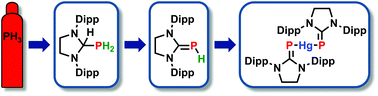
PH3 reacts with the in situ generated N-heterocyclic carbene DippNHC* (DippNHC* = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene) to give the phosphanyl–imidazolidine [DippNHC*-H]-[PH2]. Upon treatment with an ortho-quinone, [DippNHC*-H]-[PH2] is dehydrogenated to give the parent phosphinidene–carbene adduct DippNHC*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH. Alternative routes to [DippNHC*-H]-[PH2] and DippNHC*
PH. Alternative routes to [DippNHC*-H]-[PH2] and DippNHC*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH employ NaPH2 and (TMS)3P7 (TMS = trimethylsilyl), respectively, as phosphorus sources. The adduct DippNHC*
PH employ NaPH2 and (TMS)3P7 (TMS = trimethylsilyl), respectively, as phosphorus sources. The adduct DippNHC*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH and the related adduct DippNHC
PH and the related adduct DippNHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH (DippNHC = bis(2,6-diisopropylphenyl)imidazol-2-ylidene) possessing an unsaturated NHC backbone both react with HgCl2 to give the bis(carbene–phosphinidenyl) complexes [(DippNHC*
PH (DippNHC = bis(2,6-diisopropylphenyl)imidazol-2-ylidene) possessing an unsaturated NHC backbone both react with HgCl2 to give the bis(carbene–phosphinidenyl) complexes [(DippNHC*![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)2Hg] and [(DippNHC
P)2Hg] and [(DippNHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P)2Hg].
P)2Hg].
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...