Magnetic properties of manganese based one-dimensional spin chains†
Abstract
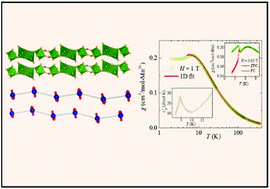
We have correlated the structure–property relationship of three manganese-based inorganic–organic hybrid structures. Compound 1, [Mn2(OH-BDC)2(DMF)3] (where BDC = 1,4-benzene dicarboxylic acid and DMF = N,N′-dimethylformamide), contains Mn2O11 dimers as secondary building units (SBUs), which are connected by carboxylate anions forming Mn–O–C–O–Mn chains. Compound 2, [Mn2(BDC)2(DMF)2], contains Mn4O20 clusters as SBUs, which also form Mn–O–C–O–Mn chains. In compound 3, [Mn3(BDC)3(DEF)2] (where DEF = N,N′-diethylformamide), the distorted MnO6 octahedra are linked to form a one-dimensional chain with Mn–O–Mn connectivity. The magnetic properties were investigated by means of magnetization and heat capacity measurements. The temperature dependent magnetic susceptibility of all the three compounds could be nicely fitted using a one-dimensional S = 5/2 Heisenberg antiferromagnetic chain model and the value of intra-chain exchange coupling (J/kB) between Mn2+ ions was estimated to be ∼1.1 K, ∼0.7 K, and ∼0.46 K for compounds 1, 2, and 3, respectively. Compound 1 does not undergo any magnetic long-range-order down to 2 K while compounds 2 and 3 undergo long-range magnetic order at TN ≈ 4.2 K and ≈4.3 K, respectively, which are of spin-glass type. From the values of J/kB and TN the inter-chain coupling (J⊥/kB) was calculated to be about 0.1J/kB for both compounds 2 and 3, respectively.

 Please wait while we load your content...
Please wait while we load your content...