Kinetics and mechanisms of reactions between H2O2 and copper and copper oxides
Abstract
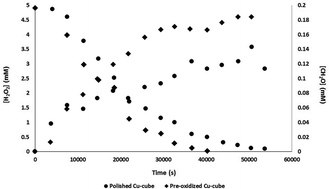
One of the main challenges for the nuclear power industry today is the disposal of spent nuclear fuel. One of the most developed methods for its long term storage is the Swedish KBS-3 concept where the spent fuel is sealed inside copper canisters and placed 500 meters down in the bedrock. Gamma radiation will penetrate the canisters and be absorbed by groundwater thereby creating oxidative radiolysis products such as hydrogen peroxide (H2O2) and hydroxyl radicals (HO˙). Both H2O2 and HO˙ are able to initiate corrosion of the copper canisters. In this work the kinetics and mechanism of reactions between the stable radiolysis product, H2O2, and copper and copper oxides were studied. Also the dissolution of copper into solution after reaction with H2O2 was monitored by ICP-OES. The experiments show that both H2O2 and HO˙ are present in the systems with copper and copper oxides. Nevertheless, these species do not appear to influence the dissolution of copper to the same extent as observed in recent studies in irradiated systems. This strongly suggests that aqueous radiolysis can only account for a very minor part of the observed radiation induced corrosion of copper.

 Please wait while we load your content...
Please wait while we load your content...