How to synthesize pure Li2−xFeSi1−xPxO4/C (x = 0.03–0.15) easily from low-cost Fe3+ as cathode materials for Li-ion batteries†
Abstract
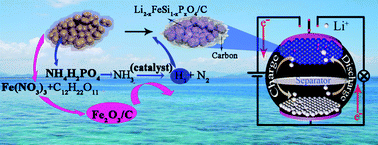
Li2FeSiO4 is a low-cost, environmentally friendly electrode material with high theoretical capacity. However, obtaining pure-phase Li2FeSiO4 on a large scale is difficult. In this study, pure Li2−xFeSi1−xPxO4/C is prepared easily by using the low cost compound Fe(NO3)3·9H2O, with the help of citric acid and appropriate ratios of NH4H2PO4 (x = 0.03–0.15). The possible mechanism of the system with NH4H2PO4 to synthesize Li2−xFeSi1−xPxO4/C is that there is a catalysis process in the system, which helps to produce H2, providing a reducing environment in every particle of the reactants guaranteeing a complete change from Fe3+ to Fe2+. The produced H2 is verified by the gas chromatography of the collected gas produced in the calcination process. The ratios of NH4H2PO4 in this system could adjust the valence of element Fe in the products. Without NH4H2PO4, an Fe2O3 impurity is formed accompanying the Li2FeSiO4. With the addition of 1 at% NH4H2PO4, the Li4SiO4 impurity accords with the objective Li2−xFeSi1−xPxO4/C. Also, Fe with zero-valence could be found as an impurity with the addition of 20 at% NH4H2PO4 due to overreduction in the system. The synthesized pure Li2−xFeSi1−xPxO4/C (x = 0.03) displayed the highest discharge capacity of 179 mA h g−1 in the first cycle, the best discharge capacity retention and the most reliable redox reversibility of the coulombic efficiency (approximately 100%), compared with the synthesized materials with Fe2O3 or Li4SiO4 impurities.

 Please wait while we load your content...
Please wait while we load your content...