A chemical method for stabilizing a new series of solid solution Pr1−xCexScO3 (0.0 ≤ x ≤ 1.0) systems†
Abstract
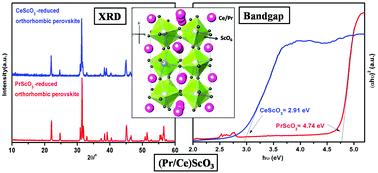
A new series of Pr1−xCexScO3 (0.0 ≤ x ≤ 1.0) compounds was synthesized by a two-step synthesis route, involving a combustion reaction followed by reduction while heating in a low partial pressure of O2, generated by a zirconium sponge that acts as an oxygen getter. For the first time, perovskite solid solution formation was observed in this series in the entire homogeneity range. These compounds were characterized using XRD, Raman spectroscopy and DRUV-visible spectroscopy. Rietveld refinement was carried out on the XRD data to determine unit cell parameters, bond lengths, bond angles along with the tilt angles for ScO6 octahedra. The analyses of the Raman shift were also in agreement with the XRD data. All compounds in this series showed a decreasing trend in the bandgap from 4.74 to 2.91 eV as a function of increasing Ce3+ concentration.

 Please wait while we load your content...
Please wait while we load your content...