Improved selectivity for Pb(ii) by sulfur, selenium and tellurium analogues of 1,8-anthraquinone-18-crown-5: synthesis, spectroscopy, X-ray crystallography and computational studies†
Abstract
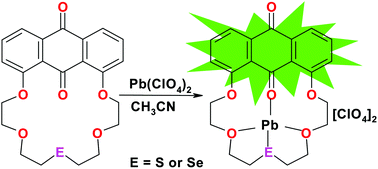
We report here a series of heteroatom-substituted macrocycles containing an anthraquinone moiety as a fluorescent signaling unit and a cyclic polyheteroether chain as the receptor. Sulfur, selenium, and tellurium derivatives of 1,8-anthraquinone-18-crown-5 (1) were synthesized by reacting sodium sulfide (Na2S), sodium selenide (Na2Se) and sodium telluride (Na2Te) with 1,8-bis(2-bromoethylethyleneoxy)anthracene-9,10-dione in a 1 : 1 ratio. The optical properties of the new compounds are examined and the sulfur and selenium analogues produce an intense green emission enhancement upon association with Pb(II) in acetonitrile. Selectivity for Pb(II) is markedly improved as compared to the oxygen analogue 1 which was also competitive for Ca(II) ion. UV-Visible and luminescence titrations reveal that 2 and 3 form 1 : 1 complexes with Pb(II), confirmed by single-crystal X-ray studies where Pb(II) is complexed within the macrocycle through coordinate covalent bonds to neighboring carbonyl, ether and heteroether donor atoms. Cyclic voltammetry of 2–8 showed classical, irreversible oxidation potentials for sulfur, selenium and tellurium heteroethers in addition to two one-electron reductions for the anthraquinone carbonyl groups. DFT calculations were also conducted on 1, 2, 3, 6, 6 + Pb(II) and 6 + Mg(II) to determine the trend in energies of the HOMO and the LUMO levels along the series.

 Please wait while we load your content...
Please wait while we load your content...