Efficient one-pot synthesis of trans-Pt(ii)(salicylaldimine)(4-picoline)Cl complexes: effective agents for enhanced expression of p53 tumor suppressor genes†
Abstract
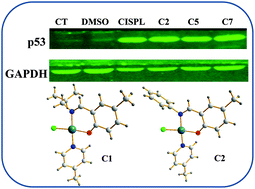
A series of trans-Pt(II)(salicylaldimine)(4-picoline)Cl complexes were synthesized in 78–87% yield using a one-pot procedure from commercially available precursors. The structures of these complexes were characterized by 1H, 19F and 13C NMR spectroscopy, HRMS (ESI) as well as single crystal X-ray analysis. Bioactivity investigations including bio-assay, time- and dose-dependent, cell cycle progression study, caspase 3 and 9 apoptosis marker assay and DNA interaction using pBR322 plasmid DNA by gel electrophoresis were performed. The results indicated that the complexes showed promising in vitro cytotoxicity in MCF-7 and A549 cancer cell lines. Moreover these complexes enhanced the expression of p53 tumor suppressor gene family members such as p63 and p73.

 Please wait while we load your content...
Please wait while we load your content...