H2 activation by a highly electron-deficient aralkylated organoborane†
Abstract
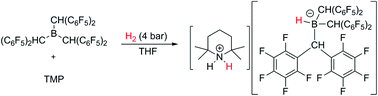
The electron-deficient and sterically bulky trialkylborane derivative tris[bis(pentafluorophenyl)methyl]borane [1, B(CH(C6F5)2)3], has been synthesised and comprehensively characterised; detailed 1H and 19F NMR studies reveal two dynamic bond rotational processes in the solution phase. Despite conventional probes (Gutmann–Beckett and Childs methods) implying that the compound has a very limited Lewis acidity, it was used to generate frustrated Lewis pairs capable of heterolytically activating H2 in ethereal solutions, which suggests that the hydridophilicity of 1 is comparable to the potent Lewis acid B(C6F5)3.

 Please wait while we load your content...
Please wait while we load your content...