Strategy to enhance solid-state fluorescence and aggregation-induced emission enhancement effect in pyrimidine boron complexes†
Abstract
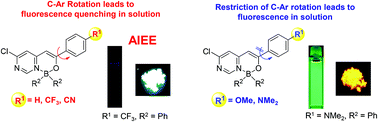
The synthesis and the solution/solid-state fluorescence properties of pyrimidine-based monoboron complexes differing in terms of the substituents [either two fluorine atoms (BF2 complex) or two phenyl groups (BPh2 complex)] on the boron atom are reported herein. Unrestricted C–Ar intramolecular rotation in the non-, trifluoromethyl-, and cyano-substituted derivatives resulted in negligible fluorescence in solution. On the other hand, methoxy- and dimethylamino-substituted analogues caused the restriction of the C–Ar intramolecular rotation and consequently resulted in relatively strong fluorescence in solution. The non-, trifluoromethyl-, and cyano-substituted derivatives showed a pronounced aggregation-induced emission enhancement effect. Dimethylamino-substituted derivatives exhibited solvatochromism in the fluorescence spectra. Substitution with BPh2 effectively enhanced the fluorescence quantum yield compared to the corresponding BF2 complexes in the solid-state.

 Please wait while we load your content...
Please wait while we load your content...