Influence of the ionic liquid cation on the solvent extraction of trivalent rare-earth ions by mixtures of Cyanex 923 and ionic liquids
Abstract
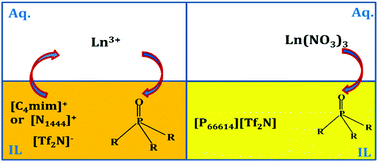
Trivalent rare-earth ions were extracted from nitric acid medium by the neutral phosphine oxide extractant Cyanex 923 into ionic liquid phases containing the bis(trifluoromethylsulfonyl)imide anion. Five different cations were considered: 1-butyl-3-methylimidazolium, 1-decyl-3-methylimidazolium, methyltributylammonium, methyltrioctylammonium and trihexyl(tetradecyl)phosphonium. The extraction behavior of neodymium(III) was investigated as a function of various parameters: pH, extractant concentration, concentration of the neodymium(III) ion in the aqueous feed and concentration of the salting-out agent. The loading capacity of the ionic liquid phase was studied. The extraction efficiency increased with increasing pH of the aqueous feed solution. The extraction occurred for all ionic liquids via an ion-exchange mechanism and the extraction efficiency could be related to the solubility of the ionic liquid cation in the aqueous phase: high distribution ratios for hydrophilic cations and low ones for hydrophobic cations. Addition of nitrate ions to the aqueous phase resulted in an increase in extraction efficiency for ionic liquids with hydrophobic cations due to extraction of neutral complexes. Neodymium(III) could be stripped from the ionic liquid phase by 0.5–1.0 M nitric acid solutions and the extracting phase could be reused. The extractability of other rare earths present in the mixture was compared for the five ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...