The first investigation of the synthetic mechanism and lithium intercalation chemistry of Li9Fe3(P2O7)3(PO4)2/C as cathode material for lithium ion batteries
Abstract
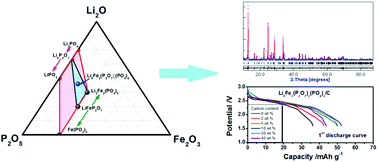
An iron-based mixed-polyanion compound, Li9Fe3(P2O7)3(PO4)2, is introduced as a possible cathode material for Li-ion batteries. Phase-pure Li9Fe3(P2O7)3(PO4)2 is successfully prepared by a sol–gel method, and its physicochemical properties are investigated in detail. Special attention is paid on making clear the variation of the phase composition with the annealing temperature and the effect of carbon coating on the electrochemical performance. Apparently phase-pure Li9Fe3(P2O7)3(PO4)2 can only be obtained in a narrow temperature range, either higher or lower annealing temperature outside this temperature range always leads to the impurity phase. The pristine Li9Fe3(P2O7)3(PO4)2 is suffering from its low electronic conductivity (10−9 S cm−1) and theoretical capacity (85 mA h g−1), it has a first discharge capacity of only 36 mA h g−1. Carbon coating is employed to improve the electrochemical performance. When the carbon content is 10 wt%, the discharge capacity of Li9Fe3(P2O7)3(PO4)2/C reaches the maximum value of 60 mA h g−1. The electronic conductivity of the composite, the exact discharge capacity of Li9Fe3(P2O7)3(PO4)2 in the composite and the capacity retention of the composite after 30 cycles vary in the same fashion with an increase in carbon content, i.e. first quickly increase and then stabilize.

 Please wait while we load your content...
Please wait while we load your content...