TQPHEN (N,N,N′,N′-tetrakis(2-quinolylmethyl)-1,2-phenylenediamine) derivatives as highly selective fluorescent probes for Cd2+†
Abstract
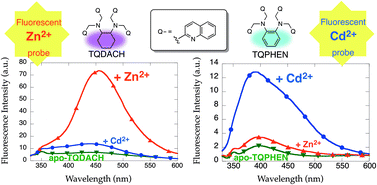
TQPHEN (N,N,N′,N′-tetrakis(2-quinolylmethyl)-1,2-phenylenediamine) and its methoxy-substituted derivatives, 6-MeOTQPHEN (N,N,N′,N′-tetrakis(6-methoxy-2-quinolylmethyl)-1,2-phenylenediamine) and TriMeOTQPHEN (N,N,N′,N′-tetrakis(5,6,7-trimethoxy-2-quinolylmethyl)-1,2-phenylenediamine), were examined as fluorescent Cd2+ sensors. Although the TQPHEN exhibits a negligible fluorescence response toward Zn2+ due to weak binding affinity in DMF–H2O (1 : 1), a 6-fold fluorescence enhancement at 392 nm was observed in the presence of 1 equiv. of Cd2+. Comprehensive X-ray crystallographic analyses of TQPHEN-Zn2+ and TQPHEN-Cd2+ complexes reveal that significant distortion in the Zn2+ complex plays a key role in the Cd2+ specificity of TQPHEN. The TriMeOTQPHEN exhibits excellent sensitivity and selectivity for Cd2+ detection (ICd/I0 = 44, ICd/IZn = 20 and LODCd = ∼10 nM (LOD = limit of detection)). On the other hand, the trans-1,2-cyclohexanediamine derivative TQDACH (N,N,N′,N′-tetrakis(2-quinolylmethyl)-trans-1,2-cyclohexanediamine) exhibits high Zn2+ specificity in the fluorescence response and extremely high Zn2+ binding affinity (Dalton Trans., 2013, 42, 9688). Subtle differences in the PHEN and DACH backbone significantly alter the stability with a specific metal and the fluorescence response of tetrakisquinoline-based fluorescent probes.

 Please wait while we load your content...
Please wait while we load your content...