Naked-eye detection and thermochromic properties of Cu(ii)-3,3′-thiodipropionate complexes with benzimidazole†
Abstract
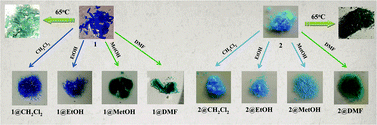
Two new coordination complexes, namely, [Cu(tdp)(H2O)(bim)3]·4H2O (1) and {[Cu(μ2-tdp)(bim)2]·4H2O}n (2) (tdp = 3,3′-thiodipropionate, bim = benzimidazole), having naked-eye sensor properties and thermochromic behaviors, were synthesized and structurally characterized using elemental analysis, IR and UV spectra, and single-crystal X-ray diffraction, powder X-ray diffraction (PXRD) and thermal analyses (TG, DTA and DTG) techniques. Complex 1 changed color from blue to dark and light green in methanol and DMF solvents, respectively, while complex 2 changed color from light blue to light green only in DMF solvent. Moreover, complex 1 can be used to detect as little as 10 percent methanol in ethanol by the naked eye. The thermochromic properties of the complexes showed that complexes 1 and 2 changed color from blue and light blue to light and dark green at 65 °C, respectively.

 Please wait while we load your content...
Please wait while we load your content...