Electrochemical fluorination of perovskite type BaFeO2.5†
Abstract
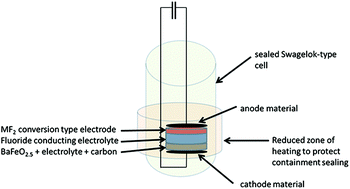
Here we report on the first electrochemical fluorination exemplarily performed on perovskite type BaFeO2.5. A cell setup of the type BaFeO2.5 II La0.9Ba0.1F2.9 II MFx (with MFx being MgF2 and CeF3) was used to perform the reaction, charging the cell up to voltages of about 4 V. Formation of a compound of approximate composition BaFeO2.5F∼0.5 was observed, in agreement with diffraction studies of the independently performed chemically fluorinated compound using F2 gas, and also possessing a capacity which is close to the theoretical capacity of the material. This new method gives an alternative towards the use of highly reactive and toxic F2 gas, and provides potential in adjusting the chemical potential for oxidative chemical fluorinations.

 Please wait while we load your content...
Please wait while we load your content...