The mechanism of transition-metal (Cu or Pd)-catalyzed synthesis of benzimidazoles from amidines: theoretical investigation†
Abstract
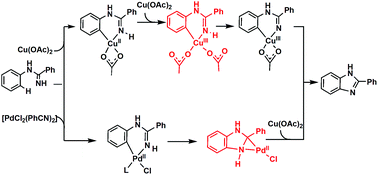
In this study, the Cu(OAc)2- and [PdCl2(PhCN)2]-catalyzed syntheses of benzimidazoles from amidines were theoretically investigated using density functional theory calculations. For the Cu-catalyzed system, our calculations supported a four-step-pathway involving C–H activation of an arene with Cu(II) via concerted metalation–deprotonation (CMD), followed by oxidation of the Cu(II) intermediate and deprotonation of the imino group by Cu(III), and finally reductive elimination from Cu(III). In our calculations, the barriers for the CMD step and the oxidation step are the same. The results are different from the ones reported by Fu et al. in which the whole reaction mechanism includes three steps and the CMD step is rate determining. On the basis of the calculation results for the [PdCl2(PhCN)2]-catalyzed system, C–H bond breaking by CMD occurs first, followed by the rate-determining C–N bond formation and N–H deprotonation. Pd(III) species is not involved in the [PdCl2(PhCN)2]-catalyzed syntheses of benzimidazoles from amidines.

 Please wait while we load your content...
Please wait while we load your content...