(Phenoxyimidazolyl-salicylaldimine)iron complexes: synthesis, properties and iron catalysed ethylene reactions†
Abstract
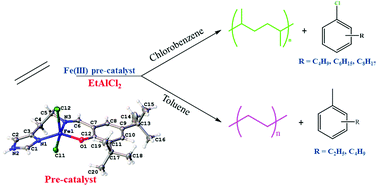
The reaction of 2-{[2-(1H-imidazol-4-yl)-ethylimino]-methyl}-phenol (L1), 2,4-di-tert-butyl-6-{[2-(1H-imidazol-4-yl)-ethylimino]-methyl}-phenol (L2) or 4-tert-butyl-2-{[2-(1H-imidazol-4-yl)-ethylimino]-methyl}-phenol (L3) with iron(II) precursors produced either iron(II) or iron(III) complexes, depending on the nature of the anions in the iron(II) precursor and the ligand. When the anion is chloride and the ligand L1, the product is [(L1)2Fe][FeCl4] (1), but when the anion is triflate (OTf−) and the ligand is L2, the product is [(L2)2Fe][OTf]2 (2). With iron(II) halides and tert-butyl groups on the phenoxy ligands L2 and L3, the iron(III) complexes [(L2)FeX2] {where X = Cl (3), Br (4) and I = (5)} and [(L3)FeCl2] (6) were formed. Complexes 1–6 were characterised by a combination of elemental analyses, IR spectroscopy and mass spectrometry; and in selected cases (3 and 4) by single crystal X-ray crystallography. The crystal structures of 3 and 4 indicated that the iron(II) precursors oxidised to iron(III) in forming complexes 3–6; an observation that was corroborated by the magnetic properties and the 57Fe Mössbauer spectra of 3 and 4. The iron(III) complexes 3–6 were used as pre-catalysts for the oligomerisation and polymerisation of ethylene. Products of these ethylene reactions depended on the solvent used. In toluene ethylene oligomerised mainly to 1-butene and was followed by the 1-butene alkylating the solvent to form butyl-toluenes via a Friedel–Crafts alkylation reaction. In chlorobenzene, ethylene oligomerised mainly to a mixture of C4–C12 alkenes. Interestingly small amounts of butyl-chlorobenzenes and hexyl-chlorobenzenes were also formed via a Friedel–Crafts alkylation with butenes and hexenes from the oligomerisation of ethylene.

 Please wait while we load your content...
Please wait while we load your content...