Synthesis, spectroscopic and electrochemical studies of phosphoryl and carbomethoxyphenyl substituted corroles, and their anion detection properties†
Abstract
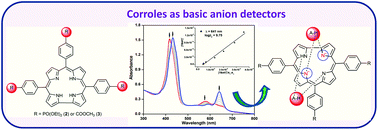
The synthesis, electrochemical studies and anion detection properties of triphosphoryl (2) and triester corroles (3) are reported and compared with triphenylcorrole (1). These corroles exhibited typical acid–base binding behaviour in CH3CN and were converted to monoprotonated and dianionic species, respectively. 2 has shown a ∼30 fold lower Keq value for monoprotonation than that of 1 in a TFA–CH3CN medium. The detection ability of these corroles was also tested in acetonitrile towards various anions. The observed spectral changes in free-base corroles (1–3) are due to anion-induced deprotonation rather than the hydrogen bonding interaction between the imino protons of the corrole moiety with anions. 2 and 3 have shown higher equilibrium constants with F− ions (4.7 × 103 fold higher for 2 and 9.7 × 103 fold higher for 3) as compared to 1 and are able to detect 0.06 μM of F− ions. The Cu(III) and Ag(III) complexes of 2 and 3 exhibited an anodic shift of ∼250 mV in first ring oxidation and ∼100–150 mV in metal centred reduction as compared to the Cu(III) and Ag(III) complexes of 1. The anodic shift in the redox potentials, lower protonation constants and lower detection limit of anions have been explained in terms of the electron-withdrawing nature of the diethylphosphite and carbomethoxy substituents at the meso-phenyl positions of the corrole ring.

 Please wait while we load your content...
Please wait while we load your content...