Zinc-specific intramolecular excimer formation in TQEN derivatives: fluorescence and zinc binding properties of TPEN-based hexadentate ligands†
Abstract
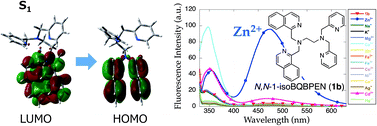
Zn2+-induced fluorescence enhancement of the TPEN (N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine)-based ligand, N,N-bis(1-isoquinolylmethyl)-N′,N′-bis(pyridylmethyl)ethylenediamine (N,N-1-isoBQBPEN, 1b), has been investigated. Upon Zn2+ binding, 1b shows a fluorescence increase (ϕZn = 0.028) at 353 and 475 nm. The fluorescence enhancement at longer wavelengths is due to intramolecular excimer formation of two isoquinolines and is specific for Zn2+; Cd2+ induces very small fluorescence at 475 nm (ICd/IZn = 10%). The excimer formation of the [Zn(1b)]2+ complex in the excited state is supported by the time-dependent DFT calculation. Neither long-wavelength fluorescence nor excimer formation is observed in the Zn2+ complex of N,N′-1-isoBQBPEN (2b). The quinoline analog N,N-BQBPEN (1a) exhibits similar but significantly smaller excimer formation. Thermodynamic and kinetic comparisons of Zn2+ binding properties of ethylenediamine-based hexadentate ligands with pyridines and (iso)quinolines are comprehensively discussed.

 Please wait while we load your content...
Please wait while we load your content...