Role of morphology in the performance of LiFe0.5Mn1.5O4 spinel cathodes for lithium-ion batteries†
Abstract
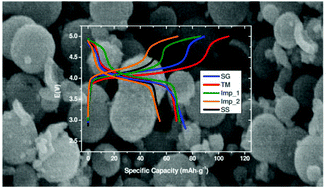
Spinel oxides with composition LiMn2−xMxO4 (M, a transition metal) are intensively studied due to their remarkable electrochemical properties. This study deals with cathode materials based on the lithium iron manganese oxide LiFe0.5Mn1.5O4 synthesized by different methods (sol–gel, in solution and hydrothermal) in order to obtain samples with various morphologies. SEM results show microspheres, composed of nanosized/submicrometer-sized subunits, microrods with a less porous surface, and finally nanoparticles that form micro-sized aggregates. The samples obtained by both solution and hydrothermal methods provided the best electrochemical behavior. In all cases, the coulombic efficiency is around 90%, and it remains constant during the tested cycles. Specific capacities remain stable between 95% and 98% of capacity retention after series of cycles in samples formed by microspheres or micro-size aggregates. These values are notably higher than those obtained for the samples with particles of heterogeneous size (49%). A LiMn1.5Fe0.5O4/Li2MnO3 composite has been prepared by the solvothermal technique in order to increase its capacity and energy density. These cells show a good cyclability at different current densities. All cells based on these LiFe0.5Mn1.5O4 cathodes recover their discharge capacity when the current density returns to C/10.

 Please wait while we load your content...
Please wait while we load your content...