A Cu(ii) complex of an imidazolium-based ionic liquid: synthesis, X-ray structure and application in the selective electrochemical sensing of guanine†
Abstract
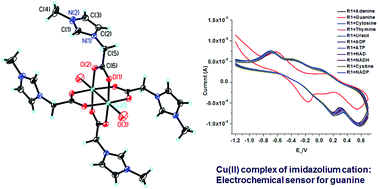
An imidazolium-based ionic liquid containing a carboxylic acid group was synthesized and complexed with Cu(II). The resulting complex R1 was fully characterized using various techniques, including IR spectroscopy and X-ray crystallography. Binding studies of the complex R1 were performed with anions and biomolecules using cyclic voltammetry, which showed no change in its voltammogram upon the addition of various anions and most biomolecules. However, a shift in the reduction peak from +0.20 to −0.15 was observed upon the addition of guanine. This selective determination of guanine by R1 was extended by using R1 as an electrochemical sensor for guanine in various voltammetric techniques, including cyclic voltammetry, LSV and DPV. The proposed sensor showed excellent reproducibility and high selectivity and sensitivity towards guanine, with a linear range of 0–20 μM and a detection limit of 45 nM.

 Please wait while we load your content...
Please wait while we load your content...