Oxygen non-stoichiometry phenomena in Pr1−xZrxO2−y compounds (0.02 < x < 0.5)
Abstract
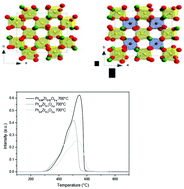
New Pr1−xZrxO2−y oxides with x < 0.5 have been prepared by co-precipitation in basic medium and annealed under air at high temperatures (T ≤ 1200 °C). Defined compositions with x = 0.02, 0.1, 0.2, 0.35, 0.40 and 0.5 have been characterized by XRD, Zr-K-edge EXAFS for the local structure, magnetic susceptibility measurements, and Pr LIII-edge XANES in order to identify the variation of the cell parameter and Zr local environment versus Zr content and Prn+ (4 < n < 3) oxidation states. The higher the Zr content, the lower the Pr valence state. The Zr amount stabilized in the distorted octahedral site is at the origin of the formation of defined compositions as discovered by Leroy Eyring et al. in the PrnO2n−2m series and the generation of oxygen vacancies stabilized in the fluorite-type network. TGA and TPR analyses help to follow the reduction properties under Ar/5% H2 and show high Pr reducible rates at low temperatures (T < 250 °C). The identification of the fluorite-type superstructure (SG: Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) of reduced compositions annealed at T = 900 °C under Ar/5% H2 shows the cationic and oxygen vacancy ordering. This feature plays a key role with Zr4+ cations stabilized in flattened octahedral sites for the generation of oxygen vacancies and the stabilization of Pr3+ in the reduced states.
) of reduced compositions annealed at T = 900 °C under Ar/5% H2 shows the cationic and oxygen vacancy ordering. This feature plays a key role with Zr4+ cations stabilized in flattened octahedral sites for the generation of oxygen vacancies and the stabilization of Pr3+ in the reduced states.

 Please wait while we load your content...
Please wait while we load your content...