Retention of single crystals of two Co(ii) complexes during chemical reactions and rearrangement†
Abstract
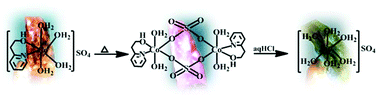
Monomeric [CoII(hep-H)(H2O)4]SO4 [1]SO4 and [CoII(hep-H)2(H2O)2](NO3)2 [2](NO3)2 have been developed from 2-(2-hydroxyethyl)pyridine (hep-H) and CoSO4·7H2O/Co(NO3)2·6H2O, respectively, at 298 K. On exposure to heat (120 °C), the light orange single crystal of [1]SO4 transforms to a pink single crystal corresponding to the neutral sulfato bridged dimeric complex [(CoII(hep-H)(H2O)2(μ2-sulfato-O,O′))2](3). However, the orange single crystal of [2](NO3)2 transforms to the single crystal of monomeric [CoII(hep-H)2(NO3)]NO3 [4]NO3 (orange) upon exposure to heat (110 °C) where one of the NO3− counter anions in [2](NO3)2 moves to the coordination sphere. The facile SCSC transformations of [1]SO4 (orange) → 3 (pink) and [2](NO3)2 (orange) → [4]NO3 (orange) involve intricate multiple bond breaking and bond forming processes without losing the crystallinity. Moreover, the immersion of the pink single crystal of 3 in 1 N HCl results in a green single crystal of ionic monomeric [CoII(H2O)6]·SO4[5]SO4, which indeed demonstrates the unprecedented unique two-step SCSC transformations.

 Please wait while we load your content...
Please wait while we load your content...